Superhydrophobic Surfaces
M.S. Bobji, R.N. Govardhan
Department of Mechanical Engineering
Indian Institute of Science
Bangalore
Hydrophobicity
Hydrophobicity refers to the physical property of a material that repels a mass of water. The word is coined from two Greek word Hydro (water) and Phobic (fear). Some of the common natural hydrophobic materials are waxes, oil and fats. Some house hold hydrophobic materials are Teflon tape and Carbon black.
Hydrophobic interaction originates from the van der Walls Interaction between water molecules and atoms or molecules of the hydrophic substances. It is characterized by surface energy of the substance in relation with the surface tension (or surface energy) of water.
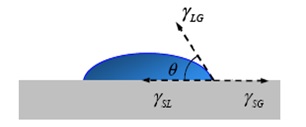 |
| Contact Angle |
cos θY= ( γSG - γSL ) / γLG
If, θY is greater than 90o then the surface is called as hydrophobic and if Y is less than 90o then the surface is called as hydrophilic surface. Maximum contact angle of 120o has been obtained for inert surfaces like Teflon (Polytetrafluoroethylene –PTFE).
Rough Surfaces
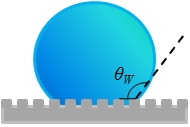 |
| "Wenzel" state |
cos θ* = r cos θY
where r is the roughness factor a ratio of the actual surface area to project area. r is greater than 1 for rough surfaces and reaches value of about 1.2 to 1.3 for fractured surfaces. However the roughness factor can be increased to very large values by various machining techniques. In nature many leaves (lotus, paper mulberry) and insects have dense hair like structures that can give rise to high roughness factors. For the Wenzel equation to be valid, water has to penetrate into the roughness.
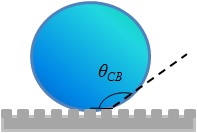 |
| "Cassie" state |
The contact angle, θ*, will be higher than the Young's contact angle if the surface is hydrophobic and will be lesser is it is hydrophilic. If the water fails to penetrate the roughness of the surface then air gets trapped in between. The contact angle in such a condition is given by Cassie- Baxter relation,
cos θ* = φs( cos θY +1) -1
Superhydrophobic surfaces
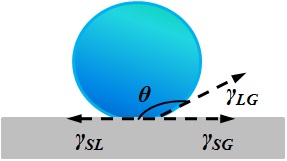 |
| Super hydrophobic - Contact angle > 140o |
Lotus effect
Lotus leaves exhibit superhydrophobicity. Water simple rolls off the leaf surface and the surface tension of the water pulls any dirt that might have been accumulated on the surface. This ability to remain clean and the ability to not get wet from the surrounding water has given rise to many a similies in Indian literature! (See Arunn’s Notebook )
Lotus leaf has tiny protuberances on its surface. It also has a waxy coating whose surface energy is such that on a smooth surface the contact angle with water is about 75o. ( Y T Cheng et al 2006 Nanotechnology, 17,1359 ) The contact angle on the lotus measured with freshly deposited water drop is about 140o. Please not that the contact angle measurement (by sessile drop method) is highly subjective and especially difficult for superhydrophic surfaces as the water drop rolls off even for a small disturbances. Mostly ig the drop is sitting on the surface quiet enough to enable measurement would mean that the water is stuck on local inhomogeneity.
To quote from Langmuir 2009, 25(20), 12120–12126