Biomechanics and Biomedical Devices
The Department houses full-fledged laboratories equipped with cell culture rooms, microscopes, instruments for mechanical characterization and biological assay, and software to conduct research on cell and tissue mechanics as well as bacterial biofilms. Additionally, there are design and prototyping facilities for developing biomedical devices. The work in biomechanics was started by Prof. G.K. Ananthasuresh with bio-micromanipulation of single cells for characterizing their mechanical response . Prof. Namrata Gundiah, with her interest in tissue and cell mechanics, substantially enhanced the scope of research in this area. She and Prof. Jaywant Arakeri collaborated to examine endothelial cells and the governing aspects of hemodynamics and mechanobiology. Prof. Aloke Kumar’s group presents another line of research with the study of biofilms. There are also other groups in the Department with interests aligned with the study of mechanics in bio- and biomedical systems.
The research initiated in biomechanics in the Department played a pivotal role in establishing a new academic centre, namely BioSystems Science and Engineering (BSSE), in IISc in 2015. Biomechanics research continues to grow synergistically in collaboration with other departments and centers in IISc and outside. It is worth noting that two start-up companies were successfully spun off from the bio-research here in the Department. One is BendFlex, which is currently pursuing an in-vitro fertilization chip using mechanical manipulation and characterization techniques developed in-house. The other is Mimyk, which specializes in medical simulation tools based on an endoscopy simulator developed here.
Biofilms
In natural and artificial environments, surfaces with moisture are often easily colonized by bacteria, which grow into dense hydrated structures called biofilms. Encased by Extra-Cellular Polymeric Substances (EPS), these biofilms are very resistant to external stresses, which make them relevant to truly diverse applications. They are relevant to the industry in many ways: fouling due to biofilms can lead to increased drag; they can cause scaling in reactors; they can lower the permeability of natural porous media. In human health, they lead to a broad range of problems ranging from tooth decay and biofouling of surgical instruments to lethal chronic infections. Thus, it is no surprise that biofilms have long been under investigation.
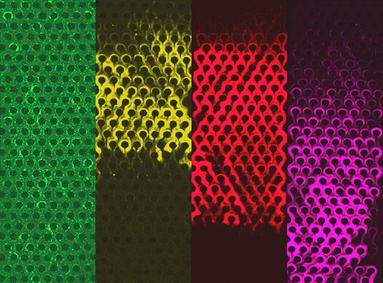
Post clogging behavior of bacterial streamers in a microfluidic device containing a micropillar array.
However, despite groundbreaking work in this direction, several aspects of biofilms remain ill understood, specifically those of an interdisciplinary nature. Growing appreciation for the relevance of this field has today brought us to an interesting juncture where we look forward to a future of multidisciplinary research in biofilms. To name a few of the important scientific challenges in this direction – interfacial interaction of microbes (bioadhesion); understanding the role of various physical influences in the formation of biofilms; design and fabrication of surfaces that can resist fouling by biofilms. Prof. Kumar’s group is in the pursuit of innovation in the interdisciplinary research area of biofilms.
Cell Mechanics
An organized and cross-linked extracellular matrix primarily contributes to tissue microstructure and material properties, which are essential in determining organismal form and function. The dynamic interplay between mechanotransduci ng proteins at focal adhesions and the substrate deformation aids in polarizing and reorienting adherent cells.
Characterizing cell-substrate interactions, involved during adhesion and migration, is a primary focus in Prof. Gundiah’s group. Using inverse approaches and the finite element method, studies showed the differences in computed forces exerted by adherent cells using substrate deformations. They also suggested a regularization method to compute traction forces which mitigated effects due to noise in the displacement data. Microfabricated ridge-pillar substrate topographies have been developed to direct cell migrations and compute tractions using beam theories. These are useful in assessing changes to the cell properties during its epithelial-to-mesenchymal transition during cancer metastasis.
To explore changes in the cell deformability associated with cancer metastasis, TGF-β, a highly expressed cytokine, is used to show increase in cell deformability and altered relaxation times that are linked to the underlying actin layouts during cell metastasis. These studies were performed using an atomic force microscope in indentation mode, and stress relaxation experiments with a modified-Hertzian contact mechanics model. Ongoing studies are aimed at identifying the specific intercellular pathway which contributes to an increase in the time-dependent cell deformability which is linked to the metastatic ability of the cell. Other studies characterize differences in the cell de-adhesion properties of cancer cells as compared to other cells using a custom-fabricated (and patented) fluid shear cell device.
Prof. Gundiah’s group has also stretched individual fibroblasts on silicone scaffolds to show the mechanistic basis for re-alignment of cells orthogonal to the direction of stretch. The orientational response of cells under uniaxial stretch was examined through a decomposition of the deformation gradient tensor into growth and elastic components, and an orthotropic fiber-reinforced solid model of the cell. The higher cytoskeletal modulus of the stretched cells, measured using atomic force microscopy, was well described using this model. They also used a multiscale approach to integrate the dynamics of stress fiber formation in the cell interior with integrin-ECM interactions under cyclic stretch. The 1D model simulates stochastic stress-dependent distribution of high and low affinity integrins at the cell membrane, tension-dependent stress fiber activation and calcium signalling variations along the cell length. Results from these simulations showed an increase in the cell tractions under cyclic stretching of the substrate which closely followed the stretch profile in the presence of a high stress fiber density.
Her group is also interested in characterizing mechanical cues that guide collective cell migrations. They showed that the presence of acto-myosin cables, bounding the enclosed cells, contribute to the creation of leader cells which direct migrations. In collaboration with Prof. Gaurav Tomar, her group is dissecting out the individual contributions of cell adhesion strength, curvatures, and cable forces on collective cellular migrations using a point particle dynamics approach. Such studies provide valuable insights into the mechanobiology of cell-substrate interactions that are important in wound healing and in cancer metastasis.
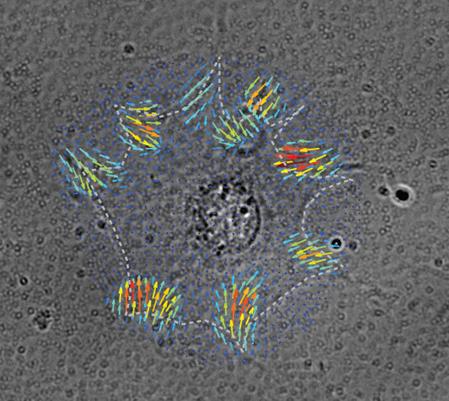
Nodal force map generated by finite element method is superposed on a brightfield image of a cell on a polyacrylamide substrate embedded with fluorescent beads. This illustrates the spatial distribution of traction forces at focal adhesions of the cell (Inside cover; Kulkarni et al., Soft Matter, 2018).
Mechanodiagnostics
Over the past 15 years, Prof. Ananthasuresh’s group has developed designs, tools, and computational techniques to manipulate (grasp, roll, squeeze, stretch, peel, probe, inject, etc.) single biological cells. The miniature compliant tools are designed such that their stiffness matches the very low stiffness (0.001 N/m to 1 N/m) of cells. This unique capability is co-developed with computational techniques for solving inverse problems in mechanics. The inverse problems posed and solved by his group enable in situ measurements of forces without using any external fields (electrical, magnetics, optical, etc.) and estimating the elastic mapping of the cell interior. Current work deals with the biological implications of changes in the mechanical response of cells. His collaborative work with Prof. Saumitra Das of Microbiology and Cell Biology (MCB) in IISc is investigating a novel hypothesis of explaining how hepatitis C virus works on a liver cell. And, there is also an investigation on how the efficacy of anti-cancer drugs can be evaluated using multimodal mechanical response. This line of work is paving the way for cell-level mechano-diagnostics of diseases. Without using any chemicals, an inexpensive portable point-of-care mechanical device capable of diagnosing diseases is a promising possibility.
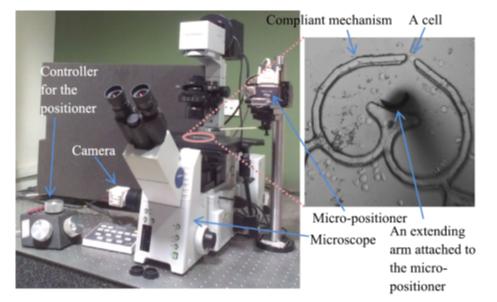
Micromanipulation setup for mechanical characterization of single biological cells
The mechanobiology and hemodynamics of endothelial cells
Namrata Gundiah & Jaywant Arakeri
Hemodynamics plays a fundamental role in the initiation, growth, and rupture of atherosclerotic plaques and aneurysms. Flow fields in arterial segments are highly three-dimensional with regions of disturbed flows; these change the direction and magnitude of shear stresses on the arterial walls through each cardiac cycle. Wall shear stress, imposed on the endothelial cell monolayer lining the arterial lumen, is a critical factor in maintaining vascular homeostasis. Deviations in the shear stress from its baseline values is recognized as the single most important parameter which serves as the mediator in geometric and material remodeling of the arterial wall.
Flow pulsatility is generally present in large arteries such as thoracic aorta and is a primary consideration when exploring effects of flow multi-directionality in endothelial mechanobiology. In contrast, flows in vessels like carotid arteries are less pulsatile and steady; they also develop aneurysms that undergo remodeling and may rupture without intervention.
Prof. Arakeri and Prof. Gundiah have collaborated to characterize the hemodynamics in curved tubes and explore links to arterial dysfunction. Their study, covering a wide range of Dean and Womersley numbers, shows the coupled roles of pulsatility and curvature on the creation of secondary flows and separations in a square-cross sectional tube with high curvature. They showed radially inward moving secondary flows have the structure of wall jets on the straight walls; their subsequent collision on the inner wall leads to a re-entrant radially outward moving wall jet. Flow separation on the inner wall, has origins in the secondary flow and not on axial pressure gradient in the tube as proposed earlier. They derived scales for the secondary flow velocities, the wall shear stress components, and the distance traversed by the secondary flow structures in the transverse plane using boundary layer equations. They next investigated flows in a patient specific model of ascending aorta with aneurysm, before and after surgery to replace the diseased section, and a patient specific model of internal carotid artery with aneurysm. Based on the secondary flows in these geometries, they showed that the unsteadiness and changing direction of the wall shear stress during a cycle is well represented using shear rosettes. These shear rosettes completely characterize the changing flows near the walls and are also linked to various other shear metrics generally used to quantify vessel dysfunction.
Using these results, they proposed a new metric, an Anisotropy Ratio (AR), based on the flow bi-directionality, which is intimately linked to the secondary flows, and demonstrate that the metric correlates with regions in the aneurysm where cell mechanobiology may be significantly altered. Ongoing studies are aimed at culturing endothelial cells within microfluidic channels and exposing them to known unidirectional laminar, oscillatory and bidirectional oscillatory flows to interrogate their response. Together, these studies are useful to probe the changes in monolayer permeability under differential flow conditions and explore the links between form and function in biological systems.
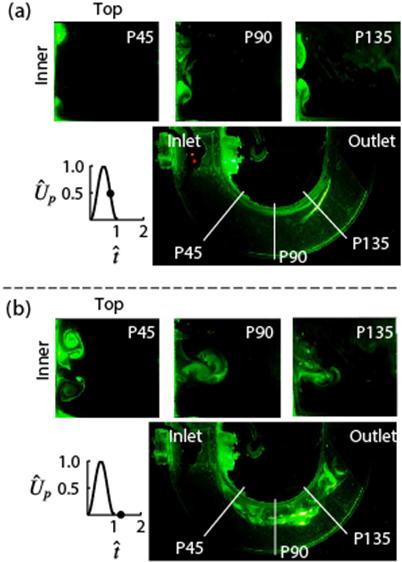
Visualizations showing the effect of pulse width on secondary flow structures at different time points in the pulse in the axial and medial planes (Krishna et al, J. Fluid Mech. 2016).
Protein design
Viewing proteins as nano-scale compliant mechanisms, Prof. Ananthasuresh applied the same optimization formulations that are used to design compliant mechanisms, to de novo protein design. The notable contributions here are making the discrete amino acid sequence space continuous and simultaneous search of the sequence and conformation spaces. Establishing a lower bound on the energy of a protein of given conformation is another significant accomplishment. He has also collaborated with Prof. Saraswathi Visweshwara of Molecular Biop hysics Unit (MBU) in IISc to apply graph theoretical methods to protein structure analysis and design.
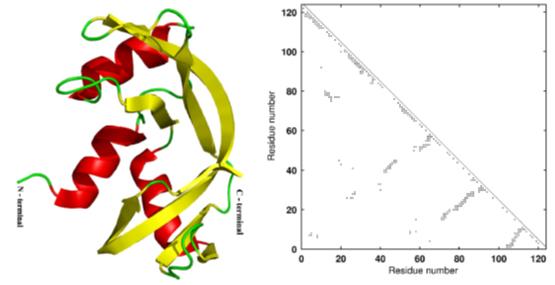
Artistic rendering and adjacency matrix of ribonuclease (7RSA) used in protein optimization studies.
Bio- and Biomedical devices
Instruments that aid in biology research and those that are useful in clinical practice are pursued in Prof. Ananthasuresh’s group. On the biology side, this group has developed miniaturized compliant tools that can manipulate and measure forces in situ. Such tools were designed, fabricated, and tested on a variety of cell lines including fibroblasts, breast cancer cells, and hepatocytes. As part of this effort, a miniature perfusion bioreactor was developed to conduct high-throughput experiments on cells cultured by emulating
in-vivo conditions with a provision for high-resolution microscopy. This is commercialized by BendFlex as Perfusion-enabled Cell Chamber (PECC) though knowhow transfer from IISc.
Biomedical devices are also developed in Prof. Ananthasuresh’s group. An endoscopic simulator, an intracranial pressure sensor, and an assistive chair for the elderly and arthritics are three examples. The endoscopy simulator was a collaborative effort with Prof. Vijay Natarajan of Computer Science and Automation, IISc. It has haptics and graphics both developed indigenously in IISc. The haptic hardware has three degrees of freedom to provide force feedback for intubation, longitudinal probing of the gast rointestinal (GI) tract, and rotational maneuver of the endoscopy tube. Graphical visualization of the GI tract with accurate anatomical features and realistic texture were also developed. Both were done in collaboration with gastroenterologists. Mimyk, a spinoff company incubated in IISc, is not only commercializing the endoscopy simulator but is also extending this as a platform to develop other medical simulators.
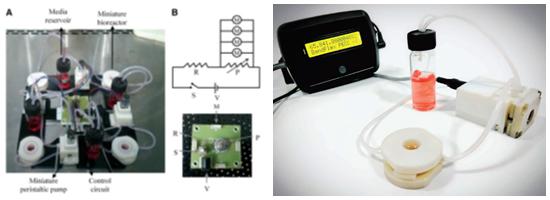
Miniature perfusion bioreactor: (left) laboratory version; (right) BendFlex’s commercial version.

Endoscopy simulator developed in IISc and incubated by IISc start-up, Mimyk.
Intracranial pressure sensor, which measures the pressure in the brain tissue, is a micromachined piezoresistive sensor developed in collaboration with Prof. Navakanta Bhat and Prof. K.N. Bhat of the Centre for Nano Science and Engineering (CeNSE) in IISc as well as Dr. Umamaheswara Rao of National Institute for Mental Health and Neuro Science (NIMHANS), Bengaluru. Design, fabrication, and packaging protocols of this sensor were transferred to SITAR, a DRDO facility for mass production. Efforts are also underway to commercialize this sensor after clinical testing.

A packaged intracranial pressure sensor and the electronic monitoring system.
Two other efforts are focused on assistive devices and both have clinical collaborators. One is with Dr. Medha Rao (Ramaiah Medical College, Bengaluru) and Dr. Pretesh Kiran (St. John’s Medical College, Bengaluru), both geriatricians; the other is with an endocrinologist, Dr. Pawan Belehalli (Karnataka Institute for Endocrinology Research, Bengaluru). With guidance from geriatricians, a novel all-mechanical assistive chair is developed to help those who have difficulty with sitting in and rising from a chair.
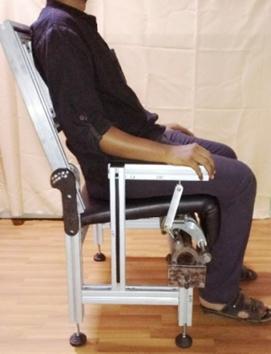
An assistive chair using a novel compliant hinge mechanism with customizable torque-angle characteristics for the elderly and arthritics.
A compliant hinge mechanism is developed to provide customized torque for an individual based on weight and height. This work, with collaboration from Prof. K.V.S. Hari (Electrical Communications Engineering) and Prof. Dibakar Sen considered an actuated chair to decide on what torque profiles are actually helpful for the elderly and paid due attention to ergonomics. Self-offloading diabetic footwear project is built upon specially designed arches and shells that undergo snap-through sequentially to offload the pressure on the sole, as determined by clinical data of patients.
Laparoscopic Apparatus
An improved laparoscopic tool is developed by Ghosal’s group that overcomes the lack of dexterity in current minimally invasive laparoscopic surgery to a large extent. The new tool has an additional joint near the cutting/grasping end and one can actuate this joint using a finger as normally done for other degrees of freedom. As shown, the rotation of the cutter/grasper is about the inclined axis and is thus different from existing articulated tools. The additional joint allows the surgeon to approach the organ/tissue or the object being cut/grasped etc. at different angles and not only from one direction as in existing laparoscopic tools. The increased dexterity allows the surgeon to a) avoid obstacles and other organs, b) access the surgery area more easily, c) provide a better viewing of the surgery area, d) enable fine motion of the cutter/grasper, and d) enable more complex suturing movements and operations. The effect of the additional joint is similar to the bending of fingers. The resulting flexibility and increased dexterity is expected to significantly reduce surgery time and stress for the surgeon. An important feature of the new design is that the degrees of freedom available in existing tools are in no way constrained or affected by this extra joint, and due to the modular nature of the design, the end-effector can be changed easily.

Prototype 1 of the improved laparoscopic tool with a cutter as end-effector.

Prototype 2 of the improved laparoscopic tool with a grasper as end-effector.
